Medical imaging equipment
Last updated: 03 April 2014
The initial agreement (or Self Regulatory Initiative - SRI) was presented in 2008 by COCIR, which represents the Radiological Electromedical and Healthcare IT industry in Europe. A revised agreement was presented 2012.
Scope
The scope of the agreement focuses on the industry sector on medical imaging equipment for human applications, including the following modalities (equivalent to product groups):
Computer Tomography (CT), Ultrasound X-Ray, Magnetic Resonance Imaging (MRI), Nuclear Medicine.
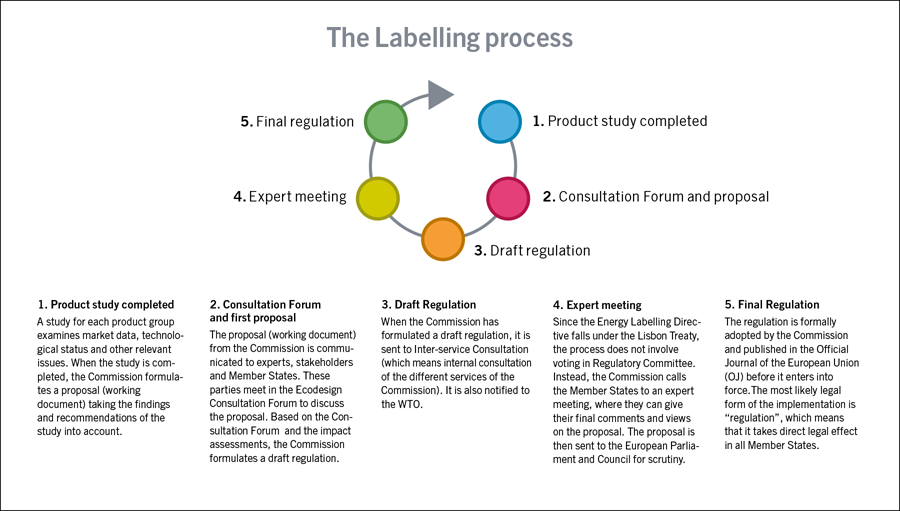
According to the proposal, the complexity of the products makes it impossible to consider all product groups at one time, since the products require specific attention to review all life cycle phases. The adopted principle is based on a 6-step approach. The aim is to target one modality per year.
eceee's viewsIn the light of relatively low aggregate energy consumption of medical imaging equipment, and the importance to safeguard the primary function of these products, eceee welcomes the self-regulatory initiative as a good intention to contribute to energy efficient product development.
For more information, view our section about Voluntary Agreements.
| Date | Process | Key documents |
|---|---|---|
| Voluntary agreement presented | Voluntary agreement for medical equipment, version 2.0 (pdf) |
|
| COCIR Steering Committee 1st Annual Forum | Invitation (pdf) |
|
New proposed voluntary agreement (pdf) |
||
| Consultation Forum | Consultation Forum |
|
Call for tenders on product study |